SKYVE has established infrastructure based on people with a high level of understanding and skill in quality control, and a system that meets Good Manufacturing Practice (GMP) standards.

Stem Cell Culture Process
We utilize advanced technology and equipment systems for stem cell isolation, large-scale culture, viability maintenance, and cryopreservation, ensuring the outstanding management of our customers’ stem cells through superior process systems.

Manufacturing Process Control
We investigate, validate, review, and document whether every step of the process—from processing, hygiene, facilities, freezing, to storage—proceeds without any issues according to established standards, making every possible effort to store our customers’ stem cells.
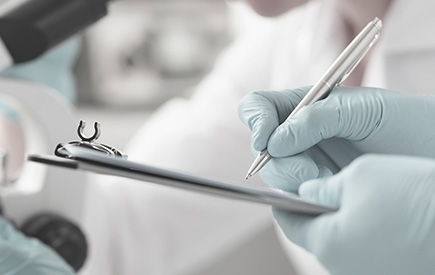
Stem Cell Quality Assurance
To consistently evaluate the safety and efficacy of stem cell therapy, we conduct analytical tests specifically designed for stem cells and continuous monitoring in compliance with the analytical methods set forth in the Korean Pharmacopoeia and the regulations of the Ministry of Food and Drug Safety (MFDS).
SKYVE’s quality control system meticulously manages all factors
that can potentially affect safety and efficacy to ensure that the best stem cells are manufactured.
It operates in strict compliance with the Good Manufacturing Practice (GMP) standards of the MFDS.
Tank system specifically designed for the long-term safe storage of stem cells

Cryopreservation in liquid nitrogen tanks at −190°C

An uninterruptible power supply ensures liquid nitrogen supply and temperature maintenance in case of a power outage

1F, 2205, Nambusunhwan-ro, Seocho-gu, Seoul,
Republic of Korea

TEL : 02-521-0521
FAX : 070-4275-5260

9:00-18:00 on weekdays, 9:00-14:00 on Saturdays,
closed on Sundays and holidays
● Consent to Collection and Use of Personal Information
We collect and use your personal information as outlined below in order to respond to your inquiry. The collected data will be used solely for the purpose of handling your request and will be safely destroyed once the purpose of use has been fulfilled, in accordance with applicable laws.
Collected Information: Name, region, affiliation, email address, contact number, etc.
Purpose of Collection: To receive and respond to customer inquiries, and to manage inquiry history
Retention Period: Until the purpose of use is achieved (or retained for the period required by relevant laws, if applicable)
This consent is required to process your inquiry, and you have the right to refuse.
However, please note that if you do not consent, you may not be able to use the inquiry service.
We refuse unauthorized collection of e-mail addresses posted on this website using e-mail collection programs or other technical devices, and please keep in mind that violations will result in criminal penalties under the Information and Communication Network Act