To improve the quality of life of patients, we study the optimization of movement with artificial joints, stem cells, and digital healthcare based on a vast amount of research data.
We are developing a wide range of products and programs, from patient-specific surgical instruments to digital healthcare solutions.
High Tibial Osteotomy (HTO) is a surgical procedure that corrects the alignment of a bent leg axis caused by medial compartment arthritis of the knee. It is performed when the condition is not severe enough to warrant joint replacement. HTO PLATE is a medical device used in performing high tibial osteotomy.

Unicompartmental Knee Replacement (UKR) is a surgical procedure that involves replacing only one side (either the medial or lateral compartment) of the knee joint with an artificial joint. It is performed when only one side of the joint, either the medial or lateral compartment, has cartilage damage. UKR is commonly performed in younger patients where the functional aspect of the joint is important.

Despite the fact that the shape of the knee varies among patients, traditional artificial joints are standardized in 8 to 10 sizes. To overcome this limitation, a product under development is custom-made artificial joints. Custom-made artificial joints are designed and manufactured taking into consideration the shape and dimensions of each patient's knee.
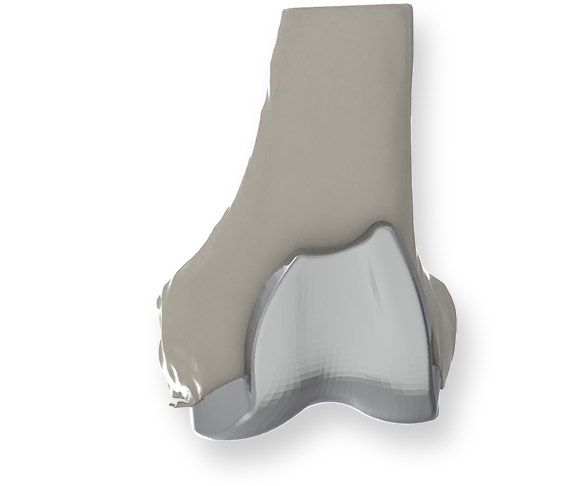
Despite the fact that the shape of the knee varies among patients, traditional artificial joints are standardized in 8 to 10 sizes. To overcome this limitation, a product under development is custom-made artificial joints. Custom-made artificial joints are designed and manufactured taking into consideration the shape and dimensions of each patient's knee.

Ultrasound therapy is a method of relieving pain using ultrasound waves. Joint ultrasound therapy can be helpful in the treatment of osteoarthritis.

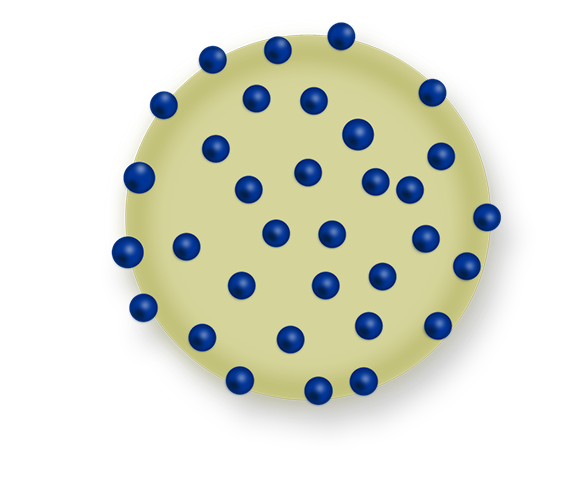
Sustained-release injectables are therapeutic agents administered through injection once every 1-3 months to reduce the frequency of medication administration or minimize side effects.
The objective is to use microspheres made of biodegradable polymer materials to release the drug slowly over an extended period within the body, thereby prolonging the duration of drug efficacy and increasing patient convenience.The goal is to develop a method for manufacturing microspheres and drug delivery technology and apply them to the development of injectable therapies for arthritis.
● Consent to Collection and Use of Personal Information
We collect and use your personal information as outlined below in order to respond to your inquiry. The collected data will be used solely for the purpose of handling your request and will be safely destroyed once the purpose of use has been fulfilled, in accordance with applicable laws.
Collected Information: Name, region, affiliation, email address, contact number, etc.
Purpose of Collection: To receive and respond to customer inquiries, and to manage inquiry history
Retention Period: Until the purpose of use is achieved (or retained for the period required by relevant laws, if applicable)
This consent is required to process your inquiry, and you have the right to refuse.
However, please note that if you do not consent, you may not be able to use the inquiry service.
We refuse unauthorized collection of e-mail addresses posted on this website using e-mail collection programs or other technical devices, and please keep in mind that violations will result in criminal penalties under the Information and Communication Network Act